
#METAL REACTIVITY TABLE SERIES#
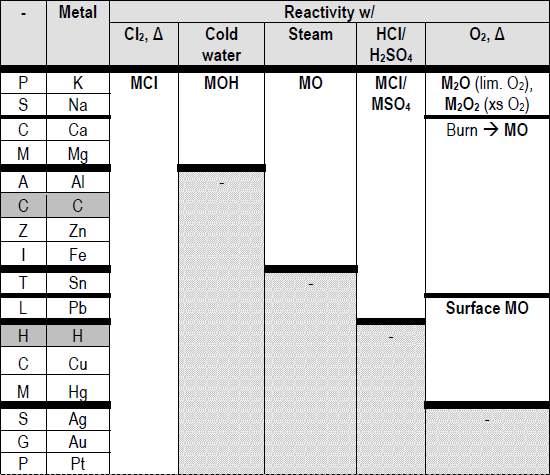
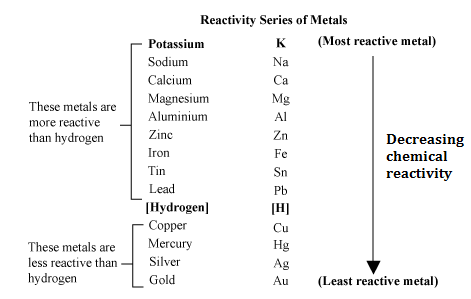
Metals at the top of the reactivity series have the ability to displace metals that are placed lower from their salt solutions.It is because, like metals, it too loses an electron and becomes a positively charged (H +) ion. Although a non-metal, hydrogen is included in the reactivity series of metals.The ease, with which a metal in solution loses electrons and forms a positive ion, decreases down the series, i.e., from cesium to platinum.Special Features of the Reactivity or Activity Series of Metals: Among the most commonly known metals, the most active cesium is at the top and the least active platinum is at the bottom of the reactivity series. The more readily metal loses its electrons, the more active it is, and the higher up it is in the reactivity series. The activity of a metal depends upon its capability to lose electrons in the solution state to form positive ions.
#METAL REACTIVITY TABLE FREE#
For example, zinc being more active than copper replaces it with copper sulfate in the solution state to form zinc sulfate and free copper. In a displacement reaction, a metal higher up in the reactivity series displaces all other metals in a compound, which lies below it. It helps us to predict whether particular metal can displace another metal from a compound or not. The total energy for the process is the sum of the energies of the individual steps.Metal activity or reactivity series finds its utility in the study of displacement reactions.Write the net balanced equation for this reaction that includes all of the steps above.second ionization energy (ga s) (+1733):.first ionization energy (gas) (+906 kJ/mol):.For zinc, write the chemical equations for the individual steps:.There are several steps to get from metal in the solid state to Zn 2+(aq). So this is really more than the loss of electrons. Zinc is defined as reacting vigorously with H+ in aqueous solution. Write the chemical equation for the oxidation of Zn.Since the experimental data indicates that zinc metal reacts vigorously with acid, there must be more to the activity of zinc than simple periodic trends. Based on the Activity Series Table, the activity of zinc is (high/low).Based on periodic trends, the activity of zinc should be (high/low) compared to other transition metals.As you go top to bottom within a column, the activity (increases/decreases).As you go left to right in the periodic table, the activity (increases/decrease).Review: Descriptive chemistry of the alkali metals and alkaline earths. The ranking of metals (and hydrogen) based on their activity is called the activity series. Propose an experiment that would allow you to determine the relative activity of any two metals.The reaction actually proceeds in the direction written, rather than the reverse.
A typical activity series reaction is the following: So, if one metal is going to lose its electrons, another species (a metal ion) has to pick them up. Metals (in their elemental form) don’t react with each other. Predict which is more active, Rb or Cs? Explain.Predict which is more active, Rb or Sr? Why?.Is this an oxidation or a reduction? Explain.Chemists are interested in trying to find the best metals to utilize.Īctivity is the tendency of a metal (and hydrogen) to LOSE electrons. In bioinorganic (and inorganic reactions), there are only a few metals that are really useful for redox.


 0 kommentar(er)
0 kommentar(er)
